1.Good safety of gelatin-free.The first lyophilized Varicella Vaccine, containing no gelatin from animals, invented and produced in China. Getting rid of gelatin from varicella vaccine can significantly decrease the ratio of anaphylaxis incidence .
2.Long validity period by good stability. The first approved varicella vaccine with 36 months of validity period in the world. Adopting BH-2 stabilizer with own IP rights (Chinese patent granted No.: ZL200910138411.6, International patent application No.: PCT/CN2009/001405) greatly enhances the stability of the product and ensures the validity period for 36 months under the storage condition.
3.Better protection with high titer and immune eff Varicella Vaccine(Live) Live Bioproducts Pharmaceutical,Live High Potency Medicine,Live Preventive Pharmaceutical,Varicella Biopharmaceutical Live Changchun BCHT Biotechnology Co.,Ltd , https://www.ccbcht.net
The first is to focus on rectification and strengthen the implementation of responsibilities. The rectification work focused on urban-rural integration areas and rural areas, with small restaurants and bars as the key locations, and focused on domestic brands and imported liquors with bulk wine, high market share, and best-selling brands, and strengthened supervision and inspection efforts. Focus on checking whether there are illegal acts of purchase, sale, use of counterfeit wines and liquors, whether the cable verification and purchase inspection system is in place, whether the labels are complete, and whether the bulk containers are in compliance with hygiene requirements. The second is to strengthen procurement management and severely punish illegal activities. Inspected the catering service units for the implementation of wine and liquor claims verification and purchase inspection systems, including the purchase account, supplier qualifications, product certification, inspection reports and certification documents, etc., and urged the company to strictly control procurement. During the inspection, it was found that the catering and service units purchased, sold, and used from unknown sources, exceeded the shelf-life, and counterfeited wine and liquor were strictly investigated and dealt with in accordance with relevant laws and regulations. The third is to increase the supervision of sampling, early detection of hidden dangers. The wine and liquor were included in the supervision of key sampling varieties, increase the frequency of sampling, detection and analysis of key indicators and risk factors. The potential hidden dangers found in the supervision of sampling inspections are early detection, early prevention, and early resolution.
The Jiangsu Provincial Bureau put forward three requirements for the special rectification work on quality and safety of wine and spirits during the catering service. The first is to strengthen organizational leadership and pay close attention to the implementation of the tasks. Combine good food safety supervision during the Spring Festival and quickly organize strengths to carry out rectification. All localities must formulate practical and feasible implementation plans to clarify the work objectives, specific tasks, work schedules, and work requirements. The second is to strengthen departmental communication and strictly submit information. Strengthen the links with the quality supervision, industry and commerce, business, and other departments. If any illegal or illegal clues involving other links are discovered in the work of rectification, the information shall be promptly reported; and the information clues reported to other departments shall be immediately investigated and dealt with. The third is to widely publicize and mobilize and strengthen social supervision. Establish and improve the public opinion supervision system and report and complaint system, and promptly report the phone to the public to accept complaints, reports and supervision from all sectors of society.
The special rectification campaign for quality and safety of wine and spirits during the catering service period will last for one month and will end at the end of February 2011. 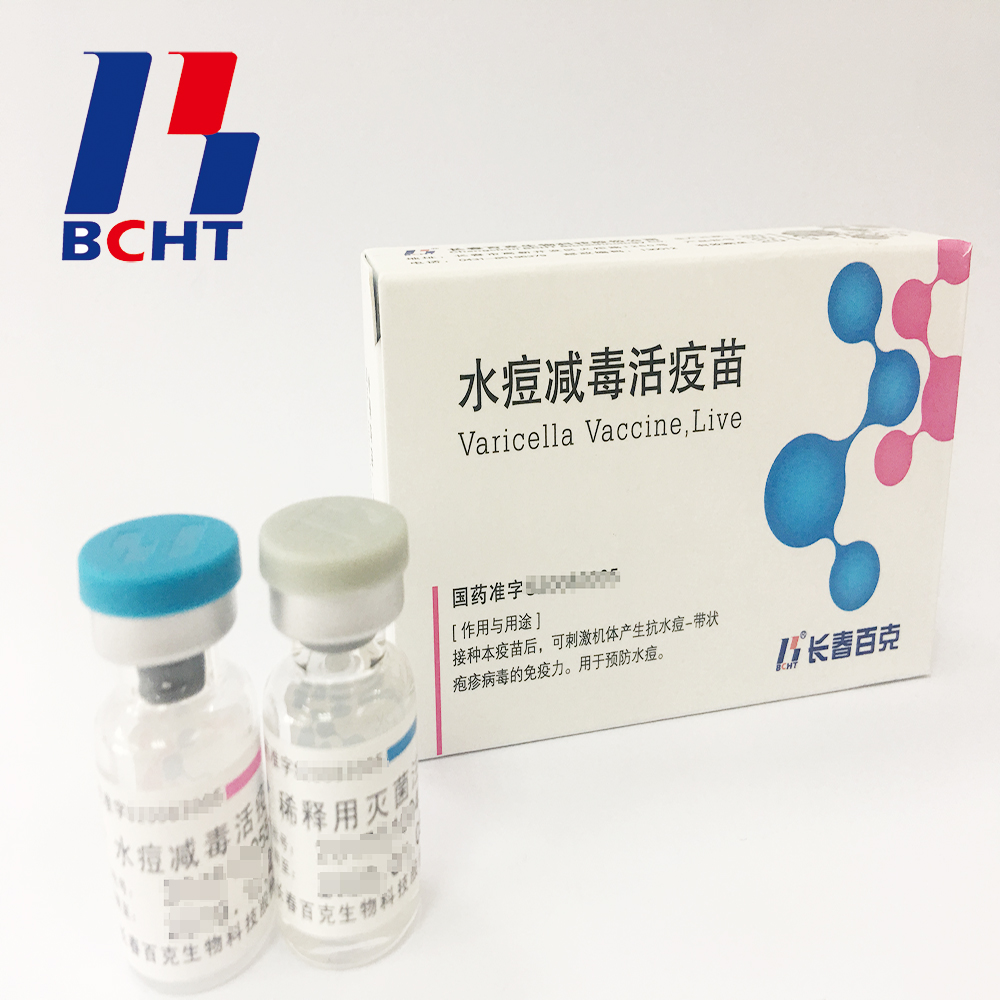 icacy. It`s documented that compared with the varicella vaccine of low titer, high titer vaccine can reduce breakthrough cases by nearly 75% in vaccination. Of all released batches of Varicella Vaccine from BCHT, the titers are not less than 10000 PFU/dose tested by National Institutes for Food and Drug Control.
icacy. It`s documented that compared with the varicella vaccine of low titer, high titer vaccine can reduce breakthrough cases by nearly 75% in vaccination. Of all released batches of Varicella Vaccine from BCHT, the titers are not less than 10000 PFU/dose tested by National Institutes for Food and Drug Control.
Jiangsu Province to Deploy Food and Wine in Wine Quality and Safety Special Renovation Work
A few days ago, the Jiangsu Provincial Food and Drug Administration issued the Implementation Plan for Special Renovation of Quality and Safety of Wine and Liquor in Food and Beverage Service in Jiangsu Province, and comprehensively deployed special rectification work on the quality and safety of wine and liquor in the catering service sector.